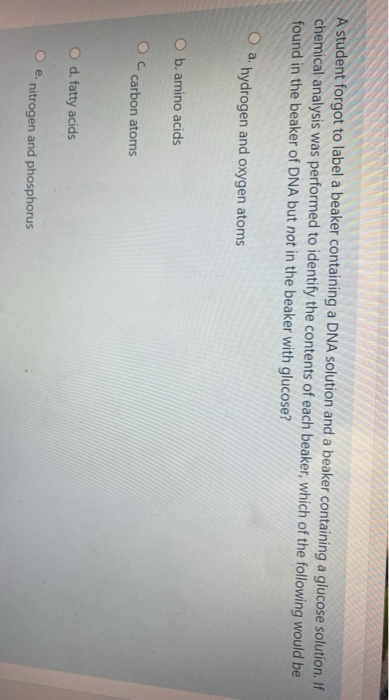
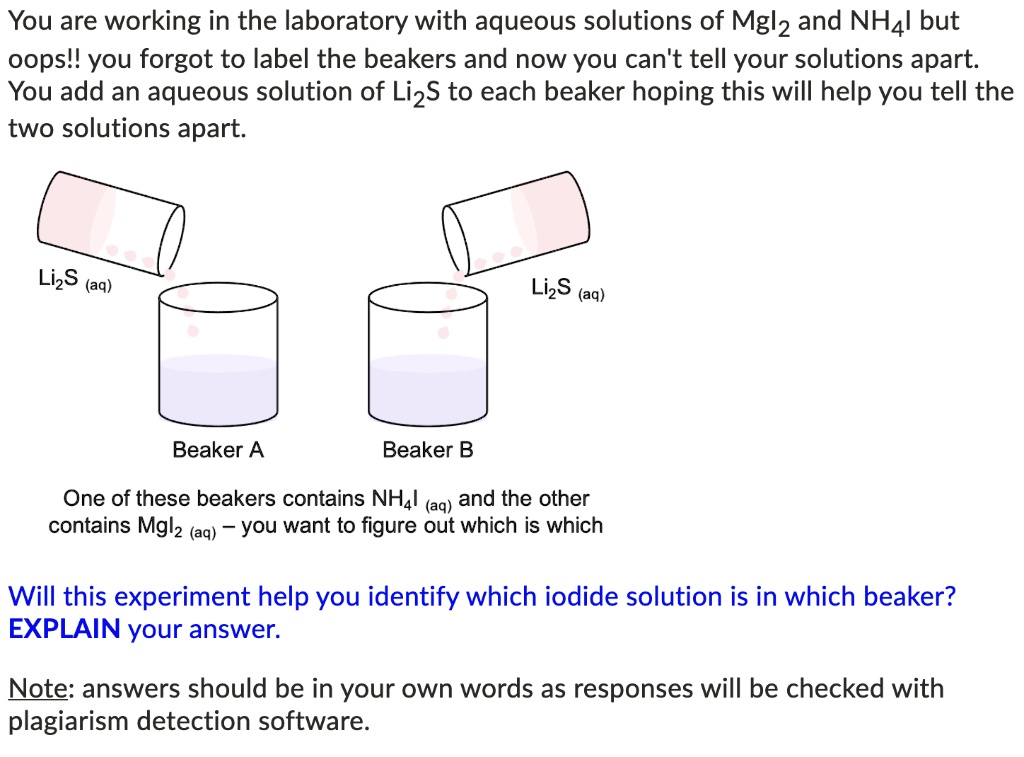
42 a student forgot to label a beaker
chem.libretexts.org › Ancillary_MaterialsThe Method of Initial Rates (Experiment) - Chemistry LibreTexts Sep 22, 2021 · Label each of these graduated cylinders appropriately. We have already begun to prepare the half of Reaction Mixture 1 listed in Table 1 for Flask I. Into the 250-mL Erlenmeyer flask containing the 10.0 mL of deionized water, add 10.0 mL of the 0.010 M \(\ce{KI}\) reagent solution and 10.0 mL of the 0.0010 M \(\ce{Na2S2O3}\) reagent solution ... coursehelponline.comCourse Help Online - Have your academic paper written by a ... Turning to course help online for help is legal. Getting assignment help is ethical as we do not affect nor harm the level of knowledge you are expected to attain as a student according to your class syllabus. Our services are here to provide you with legitimate academic writing help to assist you in learning to improve your academic performance.
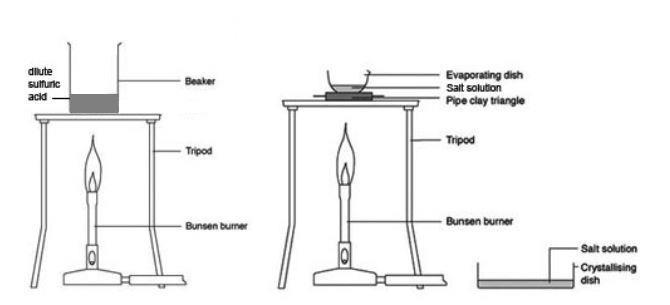
chem.libretexts.org › Courses › Los_Medanos_CollegeExperiment_729_Qualitative Testing of Amino Acids and ... Take a 600-mL or 400-mL beaker to the hood. Label it with your name. Pour some of the chromatography solvent into the beaker until it reaches a depth of about 1 cm. The liquid level must be below the origin line on your chromatography paper. Cover the beaker tightly with plastic wrap and leave it in the hood.

A student forgot to label a beaker
chem.libretexts.org › Courses › Los_Medanos_CollegeExperiment_614_Synthesis of Aspirin_1_1_2 - Chemistry LibreTexts Feb 20, 2021 · Prepare a boiling-water bath by filling a 600-mL beaker with about 400 mL of tap water. Put the beaker on the hot plate. Weigh out about 2.1 grams of salicylic acid on a piece of weighing paper. To do this, first tare a piece of weighing paper (This means to put a piece of paper on the balance and press the zero or tare button. chem.libretexts.org › Bookshelves › Organic_Chemistry4.6: Step-by-Step Procedures For Extractions - Chemistry ... Apr 07, 2022 · Figure 4.25: Student adds liquid to a separatory funnel. Pour a quantity of the extractive solvent into the separatory funnel, as indicated by the procedure (Figure 4.24c). It is unnecessary to use precise quantities of solvent for extractions, and the volumes can be measured in a graduated cylinder. chem.libretexts.org › Courses › Lansing_CommunityExperiment 7: Calorimetry - Chemistry LibreTexts Jun 18, 2019 · Place 200 mL of room temperature water from a carboy in a 250 mL beaker and set it aside for later use. Next place about 250 mL of tap water into a 400 mL beaker. Add 4-5 boiling chips into the tap water to prevent bumping. Bring the tap water to a gentle boil using a hot plate. Obtain three clean dry 18 by 175 mm test tubes. Label them runs 1 ...
A student forgot to label a beaker. › cicapairCICA SKIN CARE PRODUCTS - CICAPAIR™ - Dr. Jart US E ... Dr. Jart+ US's Cicapair™collection is the ultimate cica skin care. It soothes and calms sensitive skin and rids the complexion of redness by using tiger grass from the Asian wetlands, plus lab-tested cica skin care products you won't get anywhere else. Dr. Jart+ US's Cicapair™ uses new technology to further fortify skin with our advanced proprietary ingredients™ Jartbiome (A blend of 4 ... chem.libretexts.org › Courses › Lansing_CommunityExperiment 7: Calorimetry - Chemistry LibreTexts Jun 18, 2019 · Place 200 mL of room temperature water from a carboy in a 250 mL beaker and set it aside for later use. Next place about 250 mL of tap water into a 400 mL beaker. Add 4-5 boiling chips into the tap water to prevent bumping. Bring the tap water to a gentle boil using a hot plate. Obtain three clean dry 18 by 175 mm test tubes. Label them runs 1 ... chem.libretexts.org › Bookshelves › Organic_Chemistry4.6: Step-by-Step Procedures For Extractions - Chemistry ... Apr 07, 2022 · Figure 4.25: Student adds liquid to a separatory funnel. Pour a quantity of the extractive solvent into the separatory funnel, as indicated by the procedure (Figure 4.24c). It is unnecessary to use precise quantities of solvent for extractions, and the volumes can be measured in a graduated cylinder. chem.libretexts.org › Courses › Los_Medanos_CollegeExperiment_614_Synthesis of Aspirin_1_1_2 - Chemistry LibreTexts Feb 20, 2021 · Prepare a boiling-water bath by filling a 600-mL beaker with about 400 mL of tap water. Put the beaker on the hot plate. Weigh out about 2.1 grams of salicylic acid on a piece of weighing paper. To do this, first tare a piece of weighing paper (This means to put a piece of paper on the balance and press the zero or tare button.



























Post a Comment for "42 a student forgot to label a beaker"