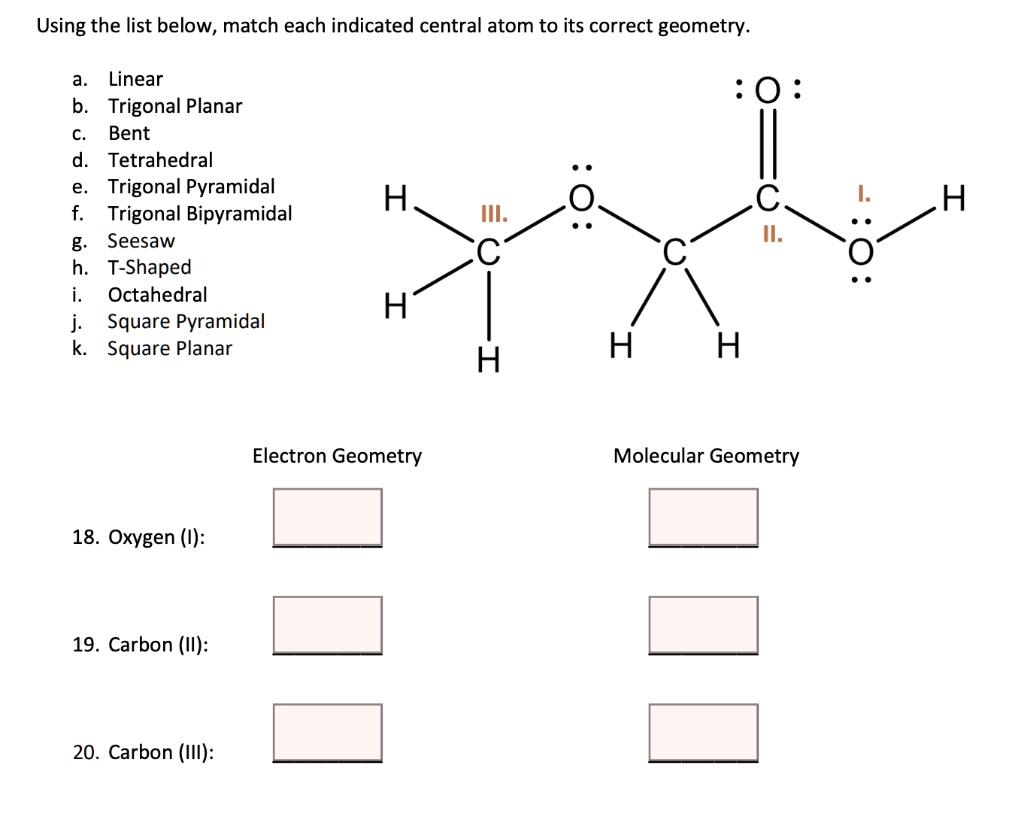
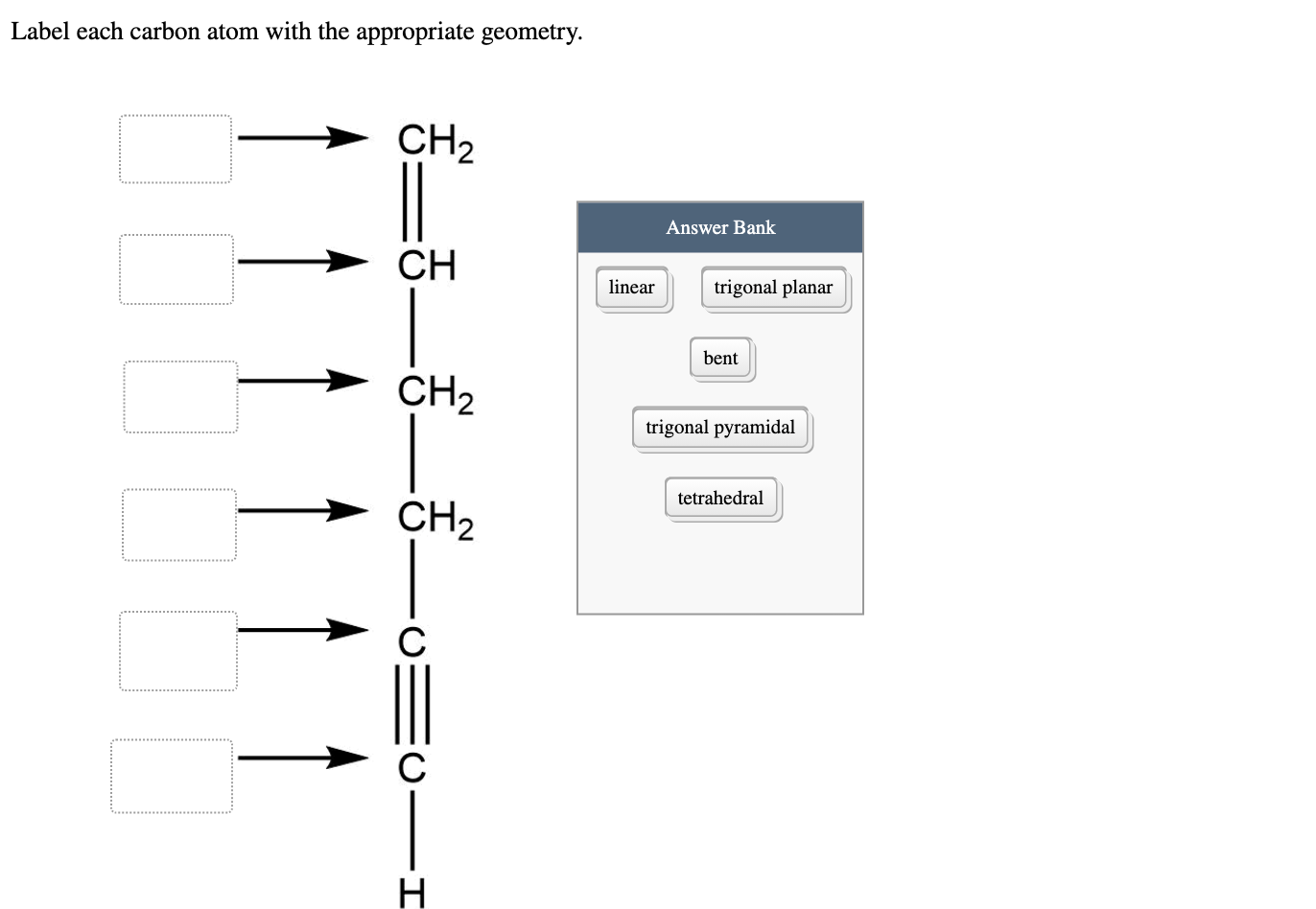
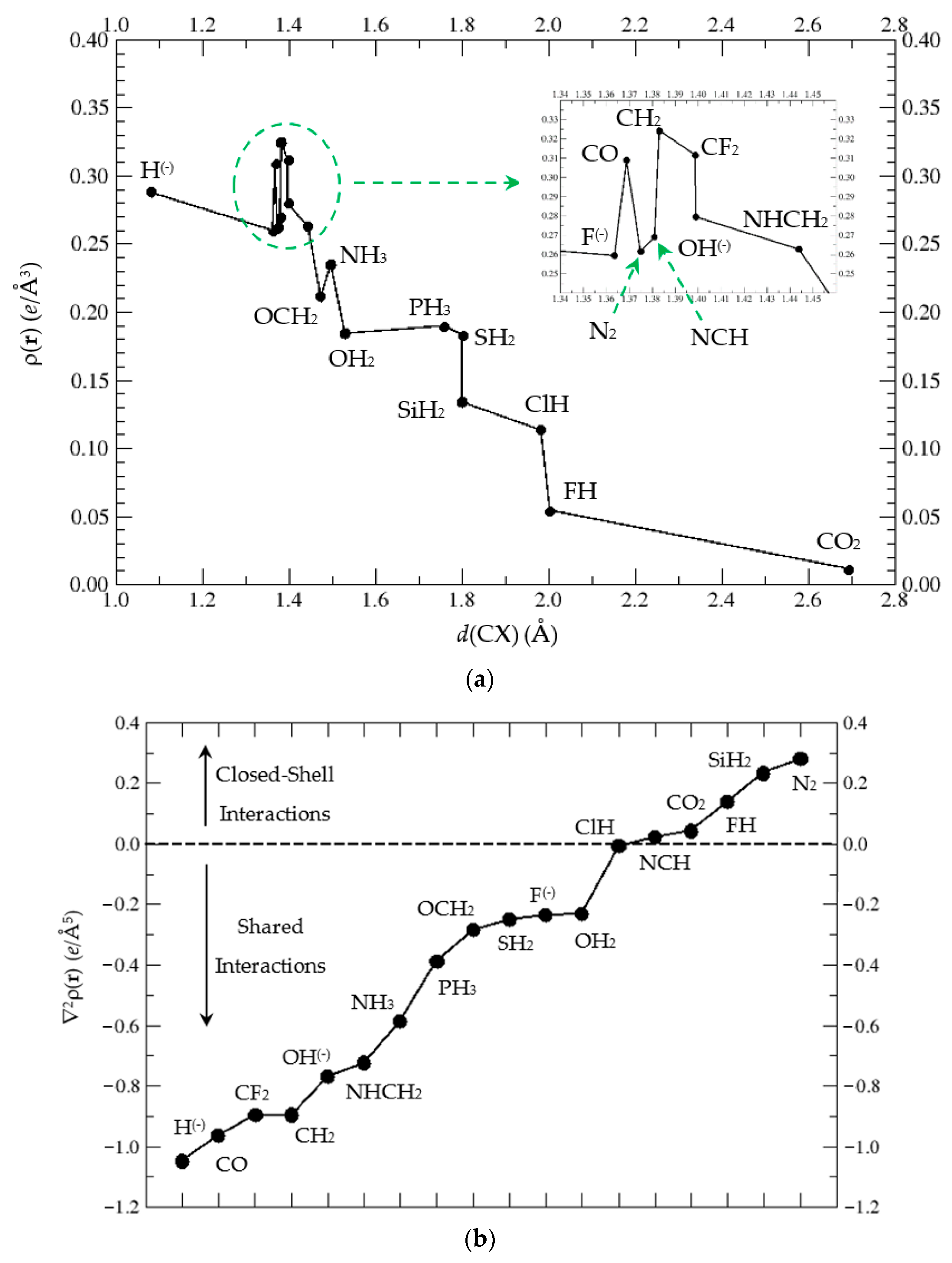
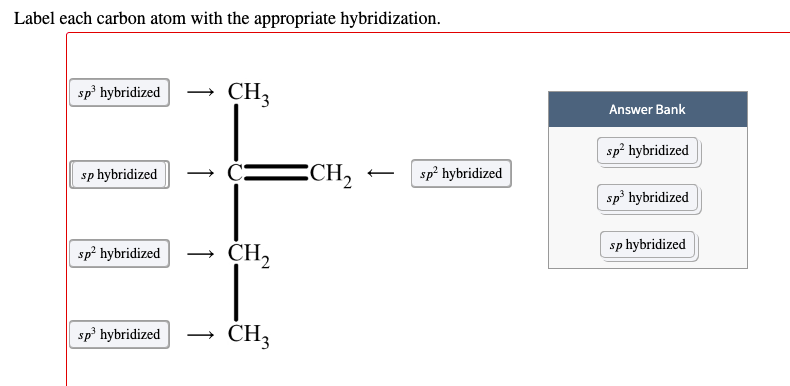
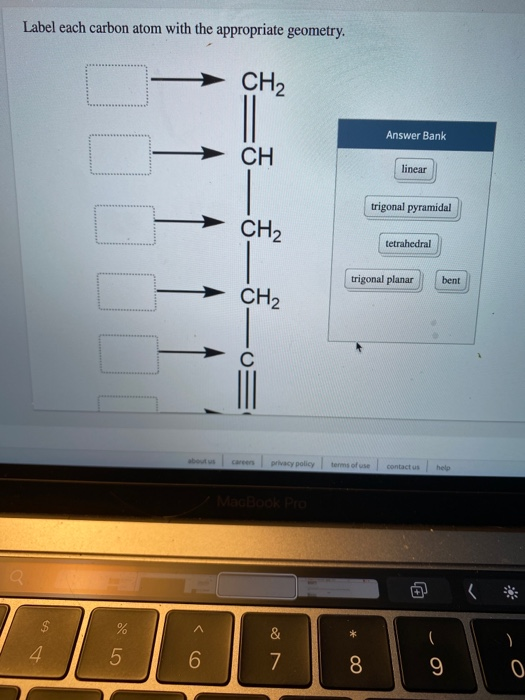
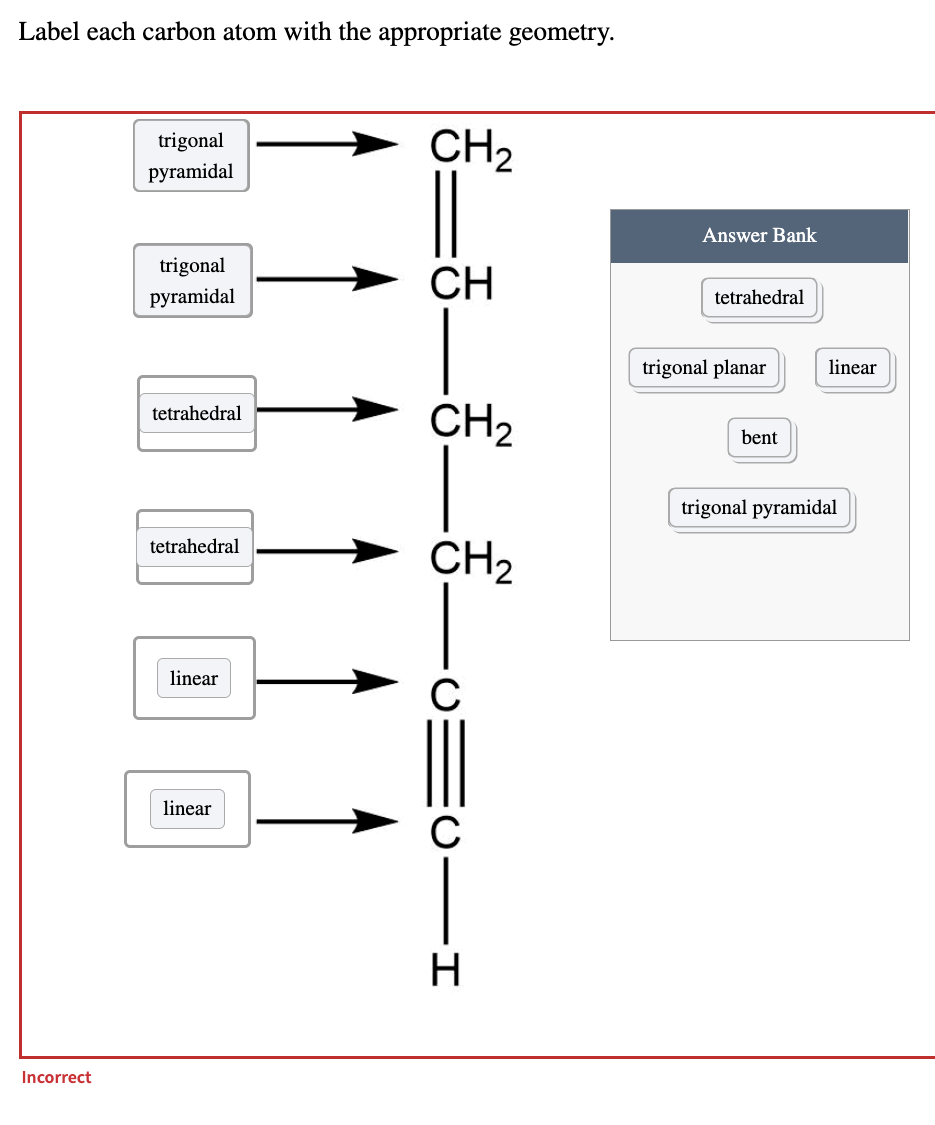
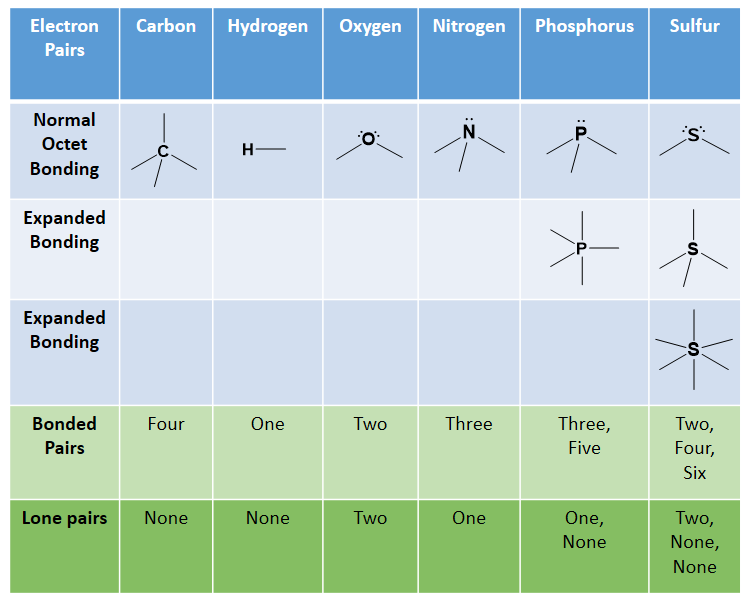
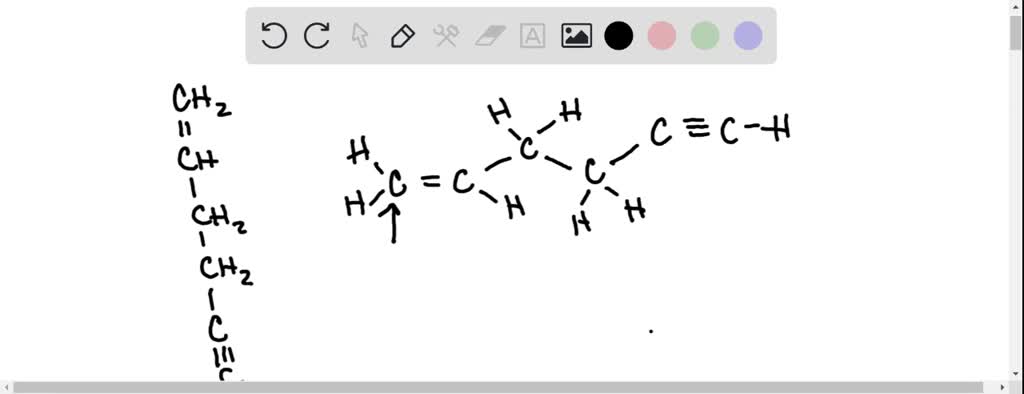
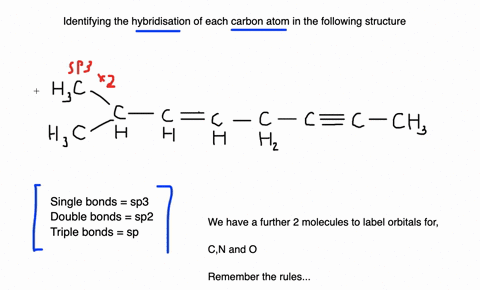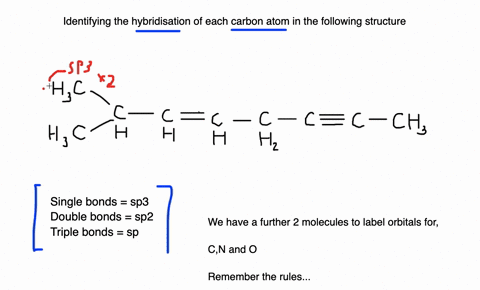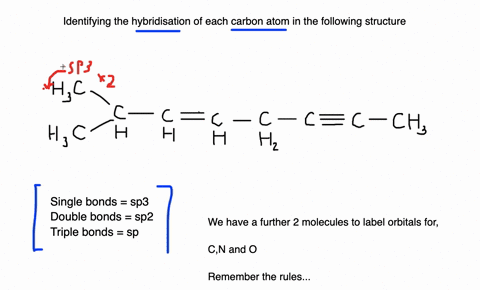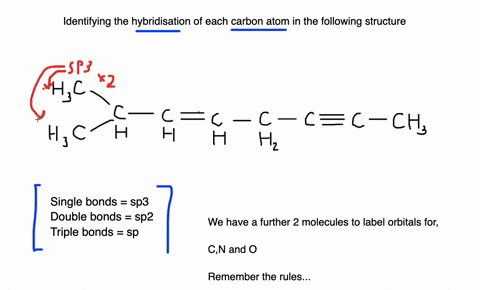
45 label each carbon atom with the appropriate geometry
Label each carbon atom with the appropriate geometry. Label each carbon atom with the appropriate geometry. ... General guidance. Concepts and reason Label each carbon atom with the appropriate geometry. The geometry at all the hybridized carbon atoms is trigonal planar and the geometry at all the hybridized carbon atoms is linear. Answer Thus, the geometry of each carbon atoms is as follows:
Label each carbon atom with the appropriate geometry. Before overlapping with the 1s orbital of hydrogen, the atomic orbitals of carbon first undergo hybridization to form hybrid orbitals. Hybrid carbon orbitals involve the formation of bonds with hydrogen. Thus, the geometry at each carbon depends on the type of hybridization. Fundamentals

Label each carbon atom with the appropriate geometry
CO2 Lewis Structure, Molecular Geometry and Hybridization CO2 Molecular Geometry. The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. As a result, there are no lone pairs of electrons, but bonding pairs of electrons also repel each other. › pmc › articlesCarbon-Related Materials: Graphene and Carbon Nanotubes in ... Aug 04, 2022 · Research on carbon crystal growth proceeded and led to the growth of thin carbon tubes [6,7]. Reports of these carbon tubes inspired Kroto and Smalley to discover fullerenes . Afterward, Huffman improved the synthesis method of fullerenes . These carbon assemblies consist of 60 atoms in which each carbon atom has sp 2 bonds. Smalley and Kroto ... CCL4 Molecular Geometry, Lewis Structure ... - Geometry of Molecules For the Lewis structure of CCl4 first, let's calculate the total valence electrons. Carbon has four valence electrons and each Chlorine atom has seven valence electrons. As there are four molecules of Chlorine, we will calculate the number of valence electrons accordingly. = 4 + (4*7) = 4 + 28. = 32 valence electrons.
Label each carbon atom with the appropriate geometry. › eharcourtschool-retiredeHarcourtSchool.com has been retired - Houghton Mifflin Harcourt Connected Teaching and Learning. Connected Teaching and Learning from HMH brings together on-demand professional development, students' assessment data, and relevant practice and instruction. label each carbon atom with its optimum C-C-C bond angle? Thanks!! Label each carbon atom with its optimum C-C-C bond angle. 180 degree 109.5 degree 120 degree 90 degree. Label each carbon atom with its optimum C-C-C bond angle. 180 degree 109.5 degree 120 degree 90 degree. en.wikipedia.org › wiki › Electron_paramagneticElectron paramagnetic resonance - Wikipedia For the microwave frequency of 9388.4 MHz, the predicted resonance occurs at a magnetic field of about = / = 0.3350 T = 3350 G . Because of electron-nuclear mass differences, the magnetic moment of an electron is substantially larger than the corresponding quantity for any nucleus, so that a much higher electromagnetic frequency is needed to bring about a spin resonance with an electron than ... Label each carbon atom with the appropriate geometry. - Transtutors Label each carbon atom with the appropriate geometry. Two binary symmetric channels (BSC) are connected in cascade as shown below. input — BSC BSC 2 output 1 Both the channels have the same transition probability and the error/cross over probability...
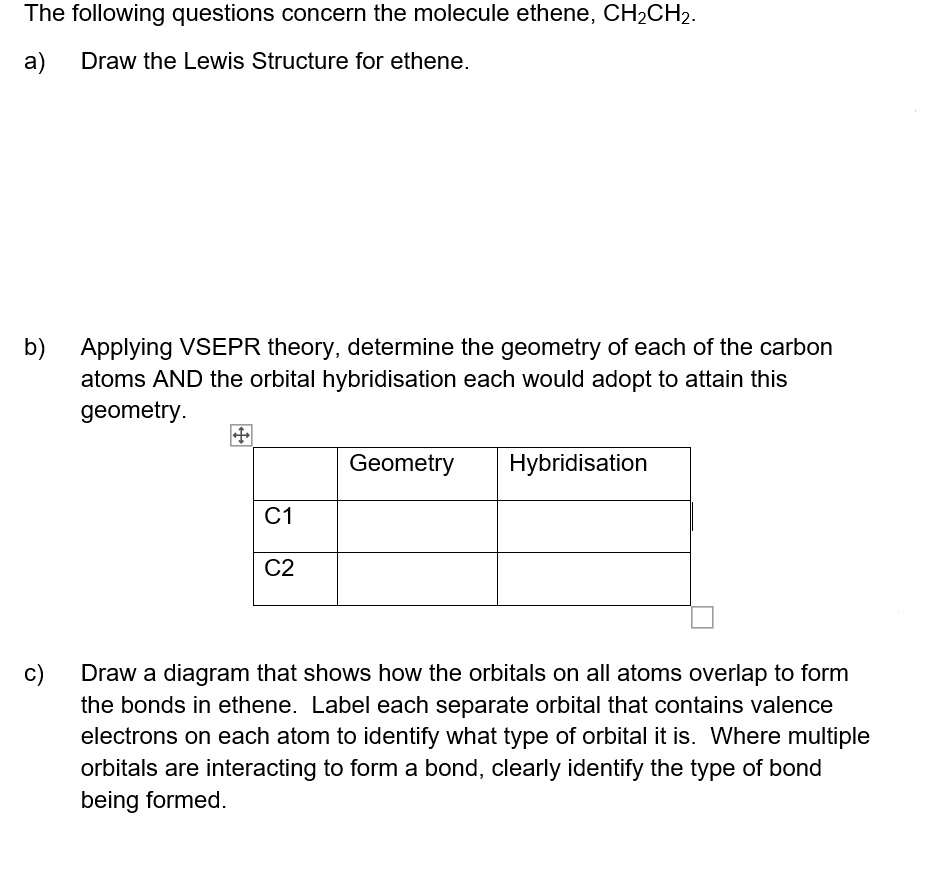
C2H4 Lewis Structure, Molecular Structure ... - Geometry of Molecules C2H4 Molecular Geometry . As it can be seen from the Lewis structure above, each Carbon atom is bonded to two Hydrogen atoms. These Hydrogen atoms repel each other on the same plane according to the VSEPR theory. Using steric numbers or the A-X-N method, we can determine the molecular structure for C 2 H 4. › questions-and-answers › how-manyAnswered: How many helium atoms in 535kg of… | bartleby Sep 19, 2022 · A: Answer: Enantiomers are the stereoisomers those are non-superimposable mirror images of each-other. question_answer Q: What mass of precipitate (in g) is formed when 20.5 mL of 0.800 M Co(NO₃)₂ reacts with 22.5 mL of… Determine Around And The Hybridization The Geometry Carbon Indicated Atoms. The molecular geometry of SOCl2 is trigonal pyramidal and its electron geometry is tetrahedral Question: Determine the hybridization and geometry around the indicated carbon atoms Also, if we calculate the steric number, the formula is as below The end result is an orbital that is mostly p shaped but it a little bit lop-sided The end result is ... Predict the approximate molecular geometry around each carbon atom of ... The electronic configuration of carbon is , this implies that carbon atom has four valence electrons.Now, in the structure left carbon atom is bonded to three hydrogens and one carbon atom, therefore, the number of bond pair is equal to four.As all the valence electrons are used in forming the bonds so, there is no lone pair left. Thus, Therefore, the orbitals that participate in hybridization ...
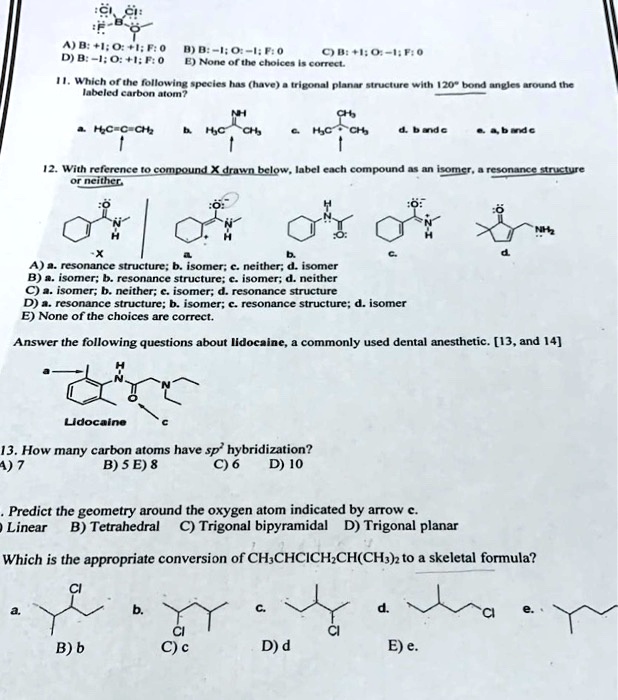
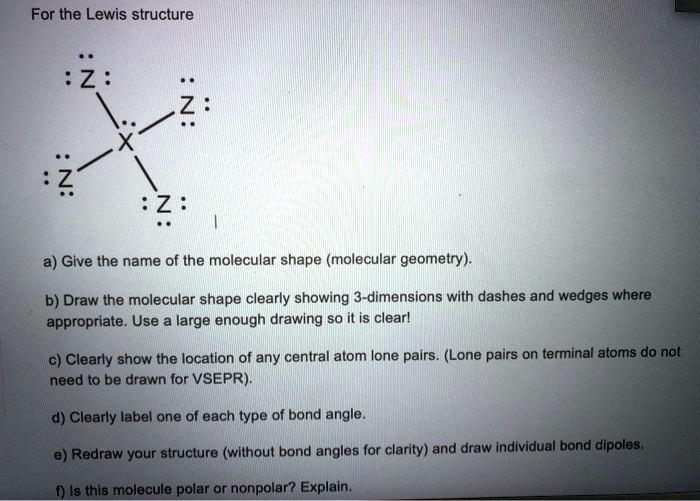
Part A Predict The Molecular Geometry Of Each Interior Atom In Acetic ... Part A Predict The Molecular Geometry Of Each Interior Atom In Acetic Acid CH3 COOH. Drag The Appropriate Labels To Their Respective Targets. Linear Trigonal Planar Bent Tetrahedral Number Of Electron Number Of Lone Molecular Geometry Pairs Atom Carbon (Left) Carbon (Right) Oxygen Groups (0 Reset Help. linear trigonal planar bent tetrahedral ... EOF Chemistry Archive | January 06, 2022 | Chegg.com Which of the following molecules contain sp2-hybridised carbon atoms? 6 6 1 2 5 Select one: Oa 2 only b. 2, 3 and 4 only O c. 2,3,4 and 5 only d. 3 and 4 only e 3, 4 and 5 only clear my choice. 1 answer ? Which of the molecules below is one of the two products of the following reaction? 1) м-СРВА НЫС 2) Н,он,о CH3 I CHE H3C H ОН ... › otm › section-3-health-hazardsOSHA Technical Manual (OTM) - Section III: Chapter 6 ... The appropriate protection guide for RF and microwave energy is that given in the American National Standard "Safety levels with respect to human exposure to radio frequency electromagnetic fields, 300 kHz to 100 GHz," ANSI C95.1; the appropriate protection guides for exposure to X-ray emission is found in the Department of Labor Occupational ...
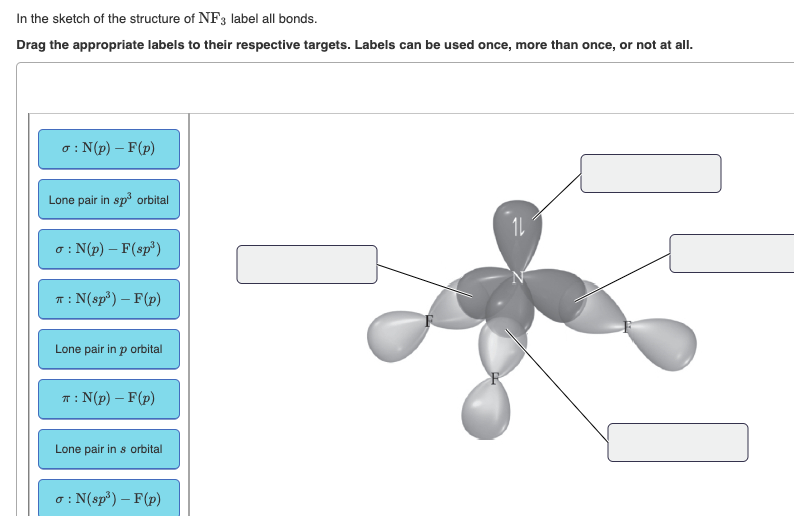
phet.colorado.edu › en › simulationMolecule Shapes - VSEPR | Lone Pairs | Bonds - PhET ... Explore molecule shapes by building molecules in 3D! How does molecule shape change with different numbers of bonds and electron pairs? Find out by adding single, double or triple bonds and lone pairs to the central atom. Then, compare the model to real molecules!
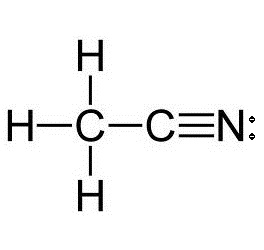
Predict the approximate molecular geometry around each carbon atom of ... Identify the hybridization of the carbon atoms present in acetonitrile by steric number, and then deduce the geometry according to the hybridization. Fundamentals Hybridization is the process of mixing the atomic orbitals of comparable energies in an atom to generate a set of new atomic orbitals which are equal in energy and shape is called ...
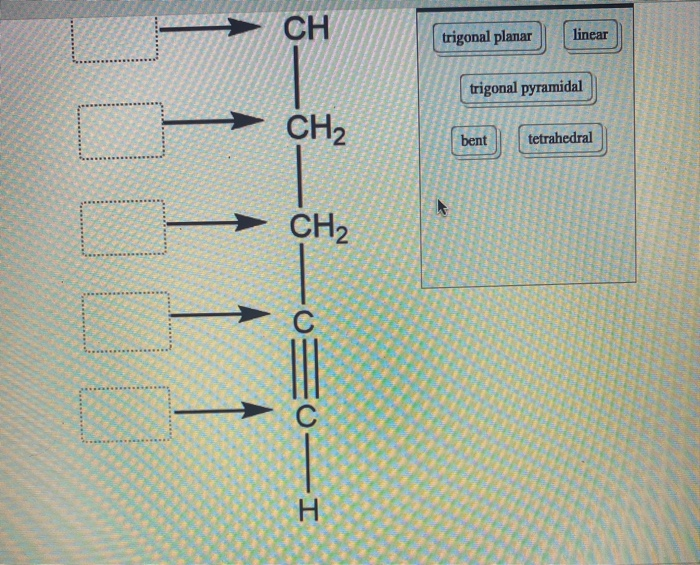
Label each carbon atom with the appropriate geometry. Transcribed Image Text Label each carbon atom with the appropriate geometry. trigonal planar CH2 trigonal planar CH trigonal planar linear tetrahedral ?H2 tetrahedral bent tetrahedral ?H2 trigonal pyramidal linear bent CH ? ? ? ?
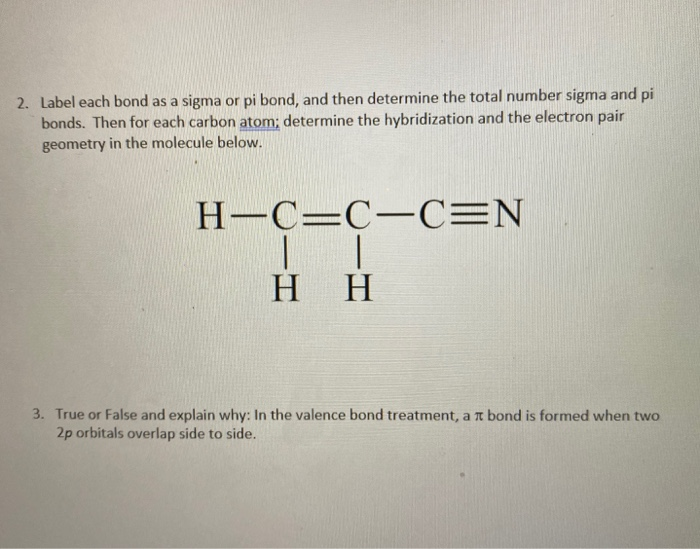
Indicated The And Geometry Carbon The Determine Hybridization Atoms ... The hydrogen-carbon bonds are all of equal strength and length, which agrees with experimental data Molecular geometry is the name of the geometry used to describe the shape of a molecule Solve: (a) The Lewis structure of NH The water molecule has two lone pairs and two bond pairs The nitrogen atom also hybridizes in the sp 2 arrangement, but ...
› topics › engineeringMetasurface - an overview | ScienceDirect Topics This one-dimensional metamaterial was composed of standard microstrip lines with complementary electric-inductor-capacitors metamaterial elements. Each metamaterial element acted as a resonator to couple microwave field from guided mode to free space. The far field radiation of the metamaterial was modified by the geometry of each element.
Atoms. Hybridization Determine Carbon Around Indicated The The Geometry ... When a molecule consists of many atoms, each carbon, oxygen, or nitrogen atom may be the center of the one of the geometries previously listed Therefore, the hybridization for Si is sp3 in SiH4 The steric number for carbon atom marked as A is 3 Determine The Hybridization And Geometry Around The Indicated Carbon Atoms Determine The ...
Lewis Structure of CH3- (With 6 Simple Steps to Draw!) - Knords Learning These pairs of electrons present between the Carbon (C) and Hydrogen (H) atoms form a chemical bond, which bonds the carbon and hydrogen atoms with each other in a CH3 molecule. Step #4: Complete the octet (or duplet) on outside atoms. If the valence electrons are left, then put the valence electrons pair on the central atom.
CCL4 Molecular Geometry, Lewis Structure ... - Geometry of Molecules For the Lewis structure of CCl4 first, let's calculate the total valence electrons. Carbon has four valence electrons and each Chlorine atom has seven valence electrons. As there are four molecules of Chlorine, we will calculate the number of valence electrons accordingly. = 4 + (4*7) = 4 + 28. = 32 valence electrons.
› pmc › articlesCarbon-Related Materials: Graphene and Carbon Nanotubes in ... Aug 04, 2022 · Research on carbon crystal growth proceeded and led to the growth of thin carbon tubes [6,7]. Reports of these carbon tubes inspired Kroto and Smalley to discover fullerenes . Afterward, Huffman improved the synthesis method of fullerenes . These carbon assemblies consist of 60 atoms in which each carbon atom has sp 2 bonds. Smalley and Kroto ...
CO2 Lewis Structure, Molecular Geometry and Hybridization CO2 Molecular Geometry. The molecular Geometry of any compound is based on the arrangement of atoms, electron pairs, and bonds. Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. As a result, there are no lone pairs of electrons, but bonding pairs of electrons also repel each other.

















Post a Comment for "45 label each carbon atom with the appropriate geometry"